Ph properties of buffer solutions lab answers – Unveiling the pH properties of buffer solutions, this comprehensive exploration delves into the intricacies of these crucial solutions, examining their composition, behavior, and diverse applications. Delving into the heart of buffer solutions, we uncover the factors influencing their pH, unravel the methods employed for accurate pH measurements, and illuminate their indispensable role in biological systems, analytical chemistry, and beyond.
Through a series of engaging experiments and detailed explanations, this discourse empowers you with a thorough understanding of buffer solutions, equipping you to confidently navigate their preparation, troubleshoot potential challenges, and harness their versatility for a wide range of scientific endeavors.
Buffer Solutions and Their Properties: Ph Properties Of Buffer Solutions Lab Answers
Buffer solutions are aqueous solutions that resist changes in pH when small amounts of acid or base are added. They play a crucial role in maintaining the pH of biological systems and are widely used in analytical chemistry.
The pH of a buffer solution is determined by the equilibrium between a weak acid and its conjugate base, or a weak base and its conjugate acid. The relative concentrations of the acid and base species determine the pH of the solution.
Factors Affecting the pH of a Buffer Solution
- Concentration of the weak acid and its conjugate base:Higher concentrations of the acid and base shift the equilibrium towards the acid or base side, respectively, affecting the pH.
- pKa of the weak acid:The pKa of the weak acid determines the strength of the acid and its ability to dissociate in solution. A lower pKa indicates a stronger acid and a higher pH in the buffer solution.
- Temperature:Temperature can affect the equilibrium constant of the dissociation reaction, leading to changes in pH.
Common Buffer Solutions
- Acetate buffer:Composed of acetic acid (CH3COOH) and sodium acetate (CH3COONa), it has a pH range of 3.7 to 5.6.
- Phosphate buffer:Composed of phosphoric acid (H3PO4) and its conjugate bases (H2PO4- and HPO42-), it has a pH range of 5.8 to 8.0.
- Tris buffer:Composed of tris(hydroxymethyl)aminomethane (Tris) and its conjugate acid (H+Tris), it has a pH range of 7.0 to 9.0.
pH Measurements in Buffer Solutions

The pH of buffer solutions can be measured using various methods:
pH Meters
pH meters are electronic devices that measure the pH of a solution by detecting the electrical potential difference between a glass electrode and a reference electrode. They provide accurate and reliable pH measurements.
Indicators, Ph properties of buffer solutions lab answers
Indicators are chemical substances that change color at a specific pH range. They are used to estimate the pH of a solution by observing the color change. However, indicators provide less precise measurements compared to pH meters.
Accuracy and Limitations of pH Measurements
The accuracy of pH measurements depends on the calibration of the pH meter, the temperature of the solution, and the presence of interfering ions.
Limitations include:
- Temperature dependence:pH meters need to be calibrated at the temperature of the solution being measured.
- Interfering ions:Some ions, such as chloride and sodium, can interfere with the pH measurement.
Applications of Buffer Solutions
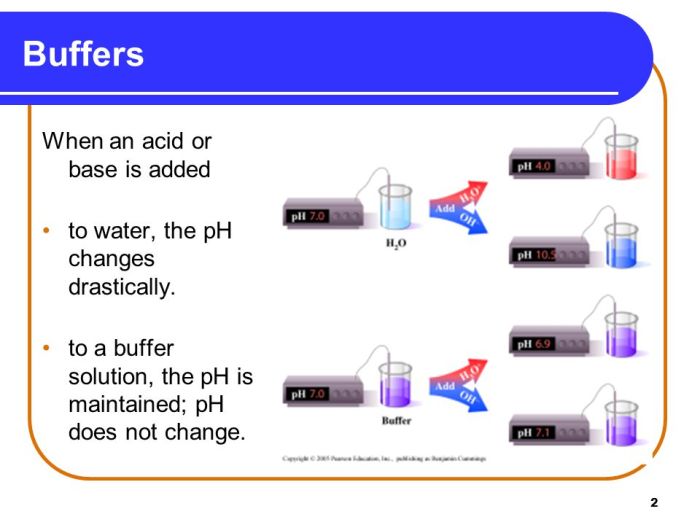
Buffer solutions have numerous applications:
Biological Systems
Buffer solutions maintain the pH of biological fluids, such as blood and urine, within a narrow range essential for optimal functioning of enzymes and other biomolecules.
Analytical Chemistry
Buffer solutions are used in:
- Titrations:To control the pH during acid-base titrations.
- Chromatography:To maintain a specific pH for optimal separation of analytes.
- Spectrophotometry:To minimize pH-dependent changes in absorbance.
Preparation of Buffer Solutions
Buffer solutions can be prepared using:
Henderson-Hasselbalch Equation
This equation calculates the pH of a buffer solution based on the pKa of the weak acid and the ratio of the concentrations of the acid and its conjugate base.
Dilution of Stock Solutions
Stock solutions of known pH can be diluted to create buffer solutions with specific pH values.
Table of Buffer Solutions
Table 1 summarizes the preparation methods for different types of buffer solutions.
| Buffer Type | Preparation Method |
|---|---|
| Acetate buffer | Combine acetic acid and sodium acetate in appropriate ratios. |
| Phosphate buffer | Combine phosphoric acid, sodium dihydrogen phosphate, and disodium hydrogen phosphate in specific proportions. |
| Tris buffer | Dissolve Tris in water and adjust the pH using hydrochloric acid or sodium hydroxide. |
Troubleshooting Buffer Solutions

Common problems include:
pH Drift
Causes:Temperature changes, contamination, or incorrect calibration of pH meter.
Solutions:Recalibrate the pH meter, ensure proper temperature control, and check for contamination.
Insufficient Buffer Capacity
Causes:Low concentration of buffer components.
Solutions:Increase the concentrations of the weak acid and its conjugate base.
Precipitation
Causes:Exceeding the solubility of buffer components.
Solutions:Use more soluble buffer components or adjust the pH to prevent precipitation.
Clarifying Questions
What are the key factors affecting the pH of a buffer solution?
The pH of a buffer solution is primarily influenced by the concentration ratio of the weak acid and its conjugate base, as well as the temperature of the solution.
How can we accurately measure the pH of a buffer solution?
The pH of a buffer solution can be accurately measured using pH meters or pH indicators. pH meters provide precise digital readings, while pH indicators offer a visual approximation of the pH value.
What are some common applications of buffer solutions?
Buffer solutions find widespread applications in biological systems, analytical chemistry, and industrial processes. They are crucial for maintaining optimal pH conditions for enzymatic reactions, calibrating pH meters, and stabilizing pH-sensitive compounds.