Cis 1 3 dimethylcyclohexane chair conformation – Cis 1,3-dimethylcyclohexane chair conformation stands as a captivating subject within the realm of organic chemistry, beckoning researchers and students alike to delve into its intricate structural and chemical properties. This comprehensive exploration unravels the molecular intricacies of cis 1,3-dimethylcyclohexane, shedding light on its conformational stability, stereochemistry, chemical reactivity, and diverse applications.
As we embark on this journey, we will decipher the molecular architecture of cis 1,3-dimethylcyclohexane, scrutinizing the spatial arrangement of its methyl groups and hydrogen atoms. Through meticulous analysis, we will uncover the factors that govern the stability of its chair conformation, contrasting it with alternative conformations such as the boat or twist-boat forms.
Structural Features
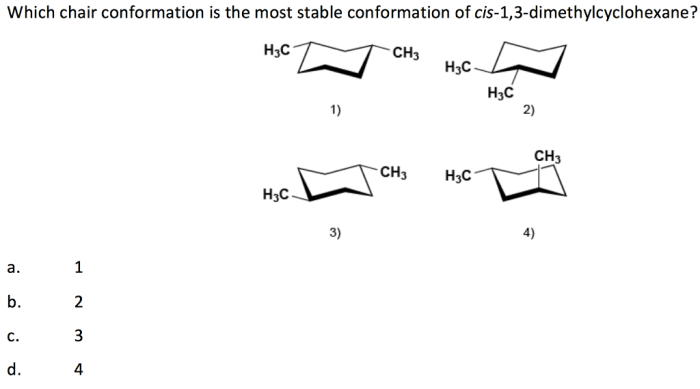
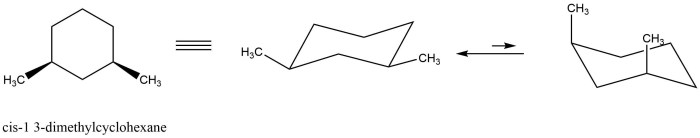
Cis 1,3-dimethylcyclohexane adopts a chair conformation to minimize steric hindrance and achieve the most stable arrangement. In this conformation, the two methyl groups are positioned on the same side of the ring, occupying axial positions. The hydrogen atoms attached to the carbons bearing the methyl groups are also in axial positions, while the other hydrogen atoms are in equatorial positions.
The chair conformation of cis 1,3-dimethylcyclohexane can be visualized using a structural diagram:

Conformational Stability
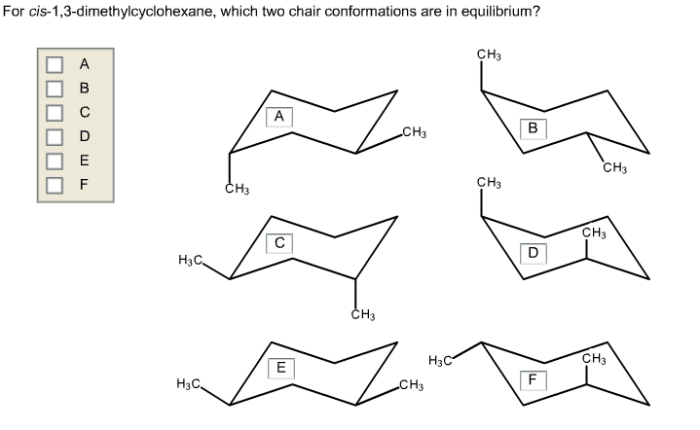
The chair conformation of cis 1,3-dimethylcyclohexane is more stable than other possible conformations, such as the boat or twist-boat conformations, due to the following factors:
- Steric interactions:In the chair conformation, the methyl groups are positioned away from each other, minimizing steric hindrance. In the boat conformation, the methyl groups are forced closer together, resulting in significant steric strain.
- Ring strain:The chair conformation has a lower ring strain than the boat or twist-boat conformations. Ring strain refers to the distortion of the cyclohexane ring from its ideal planar geometry. The chair conformation minimizes ring strain by adopting a puckered shape.
Stereochemistry
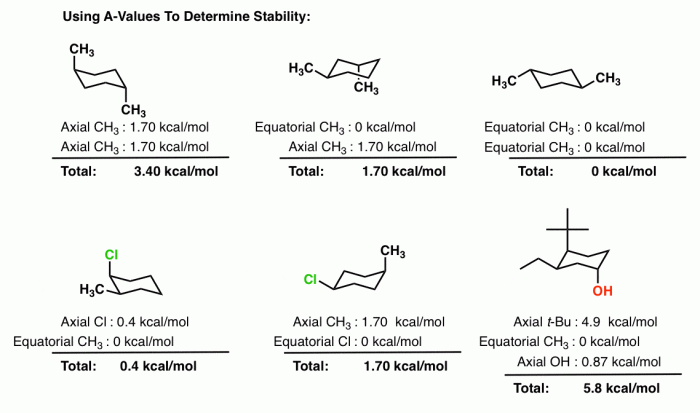
Cis 1,3-dimethylcyclohexane has two stereocenters, the carbons bearing the methyl groups. These stereocenters can have either an R or S configuration. In the chair conformation, the methyl groups are both in axial positions, which means they are both pointing up or both pointing down.
This is known as the cis configuration.
The axial and equatorial positions of the substituents have a significant impact on the overall stereochemistry of the molecule. Axial substituents are more exposed and experience greater steric hindrance, while equatorial substituents are more shielded and experience less steric hindrance.
Chemical Reactivity
The chemical reactivity of cis 1,3-dimethylcyclohexane in the chair conformation is influenced by the steric hindrance of the methyl groups. The axial methyl groups block the approach of reagents to the ring, making reactions that occur at the ring carbons more difficult.
For example, in electrophilic addition reactions, the electrophile is less likely to add to the double bond in cis 1,3-dimethylcyclohexane than in cyclohexane itself, due to the steric hindrance of the methyl groups.
Applications: Cis 1 3 Dimethylcyclohexane Chair Conformation
Cis 1,3-dimethylcyclohexane has potential applications in various fields, including:
- Solvent:Cis 1,3-dimethylcyclohexane is used as a solvent for nonpolar organic compounds, such as oils and greases.
- Fuel additive:Cis 1,3-dimethylcyclohexane is added to gasoline to improve its octane rating.
- Pharmaceutical industry:Cis 1,3-dimethylcyclohexane is used as a starting material for the synthesis of various pharmaceuticals.
Helpful Answers
What is the significance of the chair conformation in cis 1,3-dimethylcyclohexane?
The chair conformation is the most stable conformation of cis 1,3-dimethylcyclohexane due to minimized steric interactions and ring strain, providing a lower energy state compared to other conformations.
How does the presence of methyl groups influence the stability of the chair conformation?
The methyl groups contribute to the stability of the chair conformation by creating steric hindrance that prevents the molecule from adopting other conformations. This steric effect reinforces the chair conformation as the preferred and most stable form.
What are the key stereochemical features of cis 1,3-dimethylcyclohexane in the chair conformation?
In the chair conformation, the two methyl groups occupy axial positions, while the hydrogen atoms occupy equatorial positions. This arrangement results in a specific spatial orientation of the substituents, influencing the molecule’s overall stereochemistry and reactivity.